1. What is the difference between TSKgel® Super-ODS and ODS-140HTP columns?
TSKgel® Super-ODS reversed phase columns, when introduced in 1995, were the first commercially available columns packed with sub-3 micron particles. Often referred to as 2 micron packed columns, the mean particle size of the silica is 2.3 micron. The same silica lots used to prepare TSKgel® Super-ODS columns are used in the manufacture of TSKgel® ODS-140HTP columns.
There are two important differences between the two column types. First, although the same polymeric C18 bonding chemistry is used to produce TSKgel® Super-ODS and ODS-140HTP columns, the endcapping reaction on TSKgel® ODS-140HTP columns is more exhaustive than on TSKgel® Super-ODS columns (please see the following paragraph for additional details). In addition, TSKgel® Super-ODS (as well as Super-Octyl and Super-Phenyl columns) have restrictions for flow rate as well as pressure. For instance, the 4.6 mm ID TSKgel® Super-ODS columns cannot be operated above 30 MPa, nor above 4 mL/min. In contrast, the upper pressure limit for 2.1 mm ID TSKgel® ODS-140HTP columns is twice as high at 60 MPa, and there is no upper limit for the flow rate, making TSKgel® ODS-140HTP columns your TSKgel® columns of choice for use at ultra-high pressures and flow rates.
The more exhaustive endcapping reaction that is performed on TSKgel® ODS-140HTP columns means (in order of importance): 1. shorter retention, higher efficiency and better peak shape for basic compounds 2. slightly shorter retention of polar compounds 3. improved chromatography of chelating compounds.
2. What is the difference between the TSKgel® ODS-100V and TSKgel® ODS-100Z columns, and which one should I choose for my particular application?
The primary difference between the TSKgel® ODS-100V and TSKgel® ODS-100Z columns is the carbon content of the solid support. The higher carbon content of TSKgel® ODS-100Z adds additional hydrophobic characteristics beyond that present in TSKgel® ODS-100V columns, allowing the TSKgel® ODS-100Z resin to be more selective toward hydrophobic compounds. Conversely, the lower carbon content of TSKgel® ODS-100V makes it more retentive toward polar compounds.
3. What is the difference between a monomeric and polymeric C18 bonded phase?
Chemically bonded alkyl phases can be prepared through various reactions, resulting in e.g. Si-O-C-, Si-N-C- or Si-O-Si-C- linkages (the first Si atom represents the silica surface). The hydrolytic stability of these bonded phase increases in the same order. Therefore, most commercial reversed phase HPLC packing materials today are prepared from alkylsilane reagents, which result in Si-O-Si-alkyl linkages, and are stable in aqueous/organic mobile phases from pH 2 to 7.
Historically, trichloroalkylsilanes were first employed as alkyl bonding reagents in HPLC, as they were widely used in industry for the production of waxes and coatings, and are less expensive than di- and especially monochloroalkylsilanes.
When a bonded phase is prepared from a monofunctional alkylsilane reagent, the reagent can only form a single bond with a surface silanol group. The resulting bonded phase is called a monomeric bonded phase, resembling a brush type phase, in which each of the bristles represents the same chemical entity.
When using a di- or trifunctional alkylsilane reagent, the reagent can potentially attach to the silica surface with one, two or three bonds. When the reaction is performed in the presence of water, the reagent molecules can also react with other reagent molecules. The resulting structure is called a polymeric bonded phase. A polymeric bonded C18 phase resembles a three-dimensional network rather than a brush-type monomeric bonded phase.
Although this was not fully appreciated in the early days of HPLC, the formation of a polymer layer on the silica surface is difficult to control, leading to relatively large variations in retention and, to a lesser extent, selectivity from batch to batch of packing material. Despite this major disadvantage, polymeric bonded phases have advantages as well. Most importantly, polymeric C18 phases exhibit (1) higher steric selectivity for such molecules as polyaromatic hydrocarbons (PAHs), and (2) reduced silanol interaction for large biomolecules, particularly proteins and peptides. The analysis of PAHs became important in the late 1970s when EPA Method 610 recommended the separation of 16 major PAHs using HPLC with a polymeric C18 column. This method, with slight variations, has been adapted around the world.
When the bonding reaction is performed under vacuum at high temperature and in the absence of water, it is no longer possible to create a polymeric bonded phase. Under anhydrous conditions a di- or trifunctional alkylsilane molecule reacts with less than two, but on average more than one silanol group on the silica surface. Under the same conditions a monofunctional silane reagent can only react with a single silanol group. At the same time, the density of alkyl groups (from mono-, di-, or trifunctional reagents) does not change appreciably under anhydrous reaction conditions. Thus the use of a multifunctional alkylsilane reagent results in fewer residual silanol groups that are accessible to interact with solute molecules, which may result in (slightly) better peak shape and (slightly) shorter retention for polar and basic solutes.
4. What are the naming conventions for Tosoh Bioscience RPC columns?
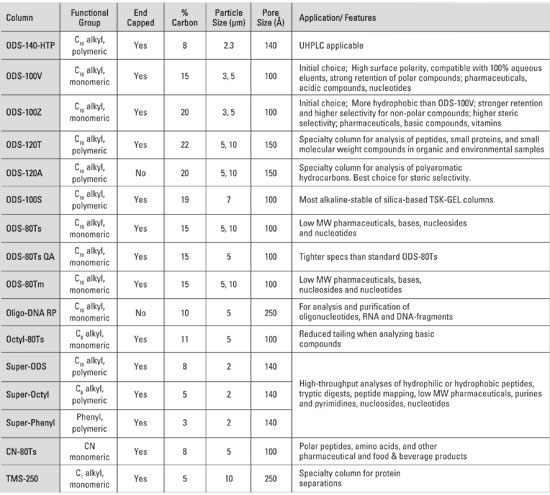
The TSKgel® designation is the company’s trademark for all liquid chromatography columns. The letters “ODS” stand for octadecylsilane or C18 bonded to a silica support material. The number 80 or 120 in the name denotes the pore size of the resin in Angstrom. For instance, the silica in TSKgel® ODS-140HTP columns has a pore size of 140 Angstrom. The letters Tm or Ts refer to an endcapped column (minimal free silanol groups). The endcapping in Ts is more exhaustive than that found in the Tm products. Tosoh Bioscience also supplies octyl (C8), phenyl, cyano (CN) and trimethylsilane (C1) columns for RPC.
Example: TSKgel® ODS-80Tm = C18 on silica with 80 angstrom pores, endcapped.
5. How do I select the optimal RPC column for my application?
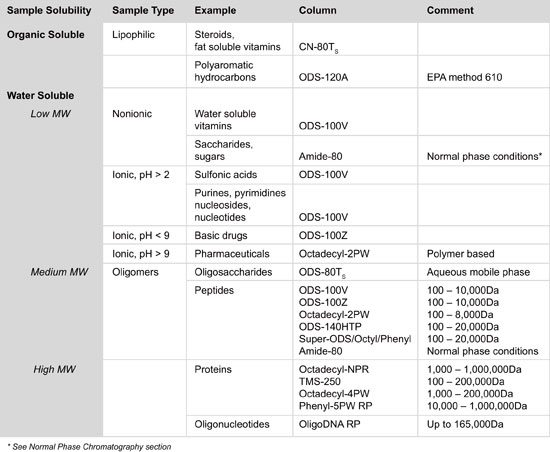
The choice of a reversed phase HPLC column is based upon the solubility and polarity of the test analytes. The more hydrophobic the sample molecule the less hydrophobic the solid support must be and vice versa. In addition to the stationary phase specifications, column configurations (including internal diameter, particle and pore size) must be considered in the choice of an appropriate column.
The stationary phase is generally made up of hydrophobic alkyl chains (e.g., -CH2-CH2-CH2-CH3) that interact with the analyte. There are three common alkyl chain lengths, C4, C8, and C18. C4-bonded phases are commonly used for proteins, while C8 and C18 are most suitable for peptides and C18 is best for small molecules. The larger protein molecule will likely have more hydrophobic moieties to interact with the column and thus a shorter chain length is more appropriate. Peptides are smaller and need the more hydrophobic longer chain length to be captured, so C8 and C18 are used for peptides as well as for small molecules. More hydrophobic molecules with extensive aromatic characteristics may be best suited to C8 ligands.
Stationary phases with high surface area and high carbon load will generally retain hydrophobic compounds longer than phases with low surface area and low carbon load. If you are analyzing macromolecules, such as peptides and proteins, a wider pore (15 -30 nm) phase usually provides better performance than a phase with small pores. High purity silicas such as type B silica, usually provide better peak shape for basic compounds than more acidic, type A, silicas.
The internal diameter (ID) of an HPLC column is a critical aspect that determines the quantity of analyte that can be loaded onto the column before overloading takes place, resulting in tailing peak shapes. Column diameter also influences sensitivity. For the same column length, the narrower the column diameter the smaller the volume in which a component elutes from the column. The most common ID is 4.6 mm, although smaller ID columns, such as 1.0, 2.0 or 2.1 mm ID, have gained popularity for use in LC-MS, as less solvent needs to be evacuated. Of course, smaller ID columns also reduce solvent usage and disposal cost.
HPLC particles come in a variety of sizes with 3 μm and 5 μm spherical beads being the most common and sub-2µm particles becoming more widely accepted. Smaller particles provide better separation efficiency, however, the pressure required for optimum linear velocity increases.
The column that works best for one application will not necessarily be the column that will work best for other applications and there is not a single column that will work best for all applications. However, this short summary will guide you in the initial selection of an appropriate column.
6. Do you have a Guide to Column Selection?
Column Selection for TSKgel® Reversed Phase Columns
7. What are “Super Series” RPLC Columns?
Super Series RPC columns are monodispersed spherical silica particles with 2 mm particle diameter and 10 nm pores. They are available with ODS, Octyl and Phenyl ligands and cover a full range of polarities (hydrophobicities). An exhaustive endcapping process assures minimal silanol activity. The small particle size resin used results in very high separation efficiency, good resolution and increased speed of analysis.
8. Does Tosoh Bioscience also offer polymeric based RPC columns?
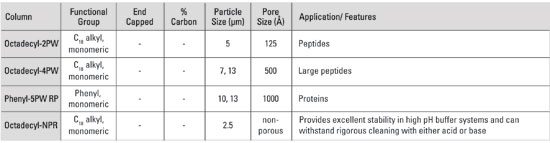
Yes. Polymer based RPC columns are stable in a pH range of 2-12. Samples can be run at high pH without any deterioration of the column plus the column can be cleaned with strong acids and bases. Tosoh Bioscience supplies these columns with non-porous resins (NPR) as well a choice of pore sizes. Column selection is dictated by the sample molecular weight and its polarity in solution.
9. What are the chemical and physical properties of TSKgel® Reversed Phase LC columns?
Properties of silica-based TSKgel® RPC columns
Properties of polymer-based TSKgel® RPC columns
10. What endfitting do I use with my particular TSKgel® Reversed Phase column?
Please visit the HPLC Columns Accessories section of the product catalog for the available fittings to be used with your TSKgel® column. Question #10 within the General FAQ category includes additional information on fittings and connectors.